In Vitro Immunology Assays
Our in vitro assays provide a precise evaluation of compound effects on immune cell function. We offer a range of customized functional readouts, including cytokine secretion, cell proliferation, activation marker expression, surface marker profiling, cytotoxicity, etc. Each assay is tailored to meet client-specific needs, with established platforms for T cell activation, T helper cell differentiation, B cell stimulation, macrophage and dendritic cell polarization, co-culture systems for immuno-oncology applications, and more.
T cells are central regulators of immune responses, orchestrating both protective immunity and immune tolerance. Their activation and effector functions are tightly balanced to eliminate infected or malignant cells while preventing self-reactivity. When this balance is disrupted, overactive T cells can drive chronic inflammation and autoimmune disease, while insufficient T cell activity can allow tumors to evade immune surveillance.
Our in vitro T cell assays model these functional responses in human systems, enabling the evaluation of therapeutic candidates that modulate T cell activation, cytokine secretion, differentiation, and effector function in the context of both autoimmune disorders and immuno-oncology applications.
B cells are essential mediators of humoral immunity, producing antibodies, presenting antigens, and secreting cytokines that shape immune responses. When tolerance mechanisms fail, dysregulated B cell activity can drive autoantibody production and autoimmune disease. B cells also interact with other immune cells, such as TFH CD4⁺ T cells, and influence the tumor microenvironment.
Our B cell assays assess therapeutic effects on B cell activation, differentiation, and function in both autoimmune and immuno-oncology settings.

Dendritic cells (DCs) are professional antigen-presenting cells that bridge innate and adaptive immunity. They capture, process, and present antigens to T cells, integrating danger signals to initiate immune responses. Dysregulated DC activity can contribute to autoimmune inflammation or impaired antitumor immunity.
Our dendritic cell assays enable the evaluation of therapeutic effects on DC maturation, cytokine secretion, and T cell priming for both autoimmune and immuno-oncology applications.
Macrophages and monocytes are innate immune cells involved in inflammation, pathogen defense, and tissue repair. Their functional state is shaped by local signals: pro-inflammatory macrophages can drive autoimmune and inflammatory diseases, while immunosuppressive macrophages can support tumor growth.
Our macrophage assays assess therapeutic effects on macrophage activation, polarization, cytokine secretion, and immune cell interactions across autoimmune and immuno-oncology research.
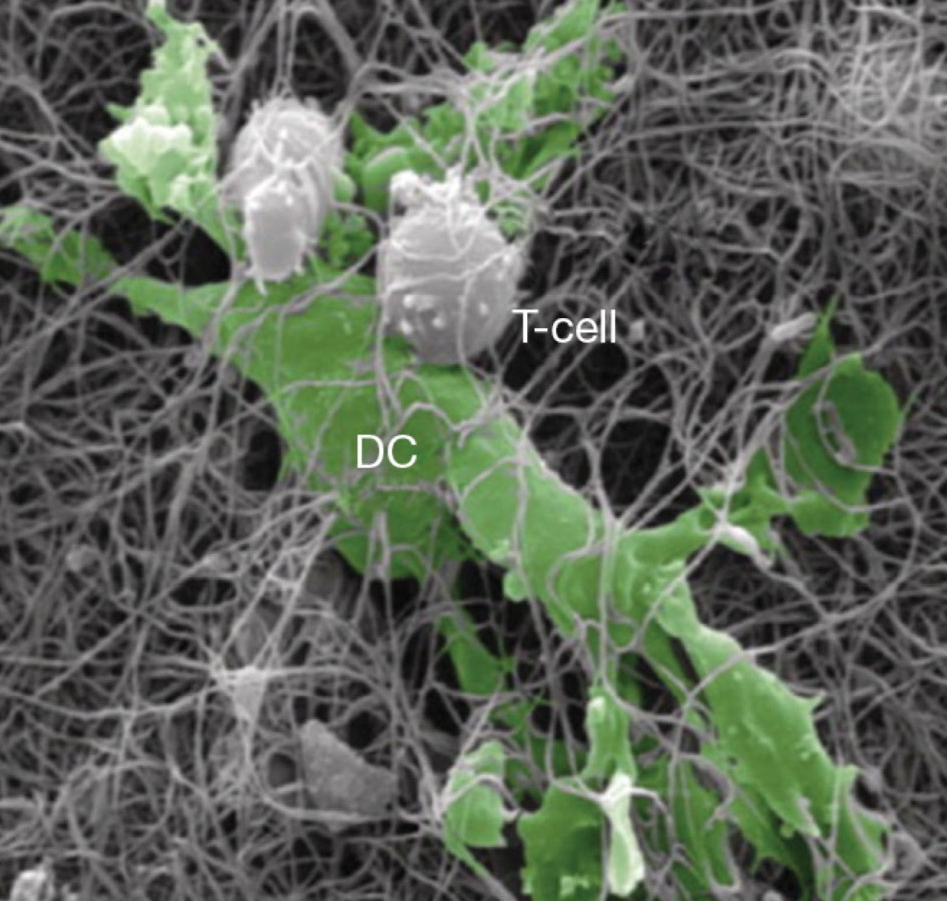
Immune cell co-culture assays model the complex interactions between different immune populations, providing insight into regulatory mechanisms and therapeutic effects. Assays such as Treg-mediated T cell suppression, M2-like macrophage–T cell suppression, and mixed lymphocyte reactions (MLR) enable the evaluation of immunosuppressive or immunostimulatory activity in vitro.
These platforms are particularly relevant for immuno-oncology and autoimmune research, allowing the assessment of how candidate therapies modulate immune cell function, crosstalk, and activation states in a physiologically relevant setting.

The tumor microenvironment often contains immune cells whose activity is suppressed or shifted toward a regulatory phenotype, allowing tumors to evade immune surveillance. Modulating these interactions is critical for effective cancer immunotherapy.
Our tumor/immune cell co-culture assays model the interplay between tumor cells and immune populations, enabling the evaluation of how therapeutic candidates restore or enhance immune activation, overcome tumor-driven immunosuppression, and promote antitumor responses in a physiologically relevant setting.

Whole blood and PBMC pSTAT assays are powerful tools to evaluate cytokine signaling through the JAK/STAT pathway in a physiologically relevant context. By measuring phosphorylation of STAT proteins in response to cytokine stimulation, these assays assess the functional status of immune signaling pathways.
They are widely used to profile pharmacodynamic responses, compare species cross-reactivity, and support dose selection for cytokine-targeting therapeutics.

We isolate and culture a wide array of human and mouse immune cells from peripheral blood, cord blood, or tumor tissues using density gradient separation and magnetic bead-based enrichment. Our team is experienced in maintaining high viability and phenotype fidelity in culture, supporting both short- and long-term experiments. Available cell types include T cells, B cells, NK cells, monocytes, dendritic cells, and macrophages and more. Cryopreserved and recallable donor lots are also available to support reproducibility and longitudinal studies.
Immunology Platforms
Our immunology platforms support a broad range of preclinical research needs, with capabilities spanning in vitro immunology assays and immune cell isolation and culturing. These platforms are designed to help clients evaluate immune-modulating compounds, characterize cell-based responses, and explore mechanism of action in a controlled, human-relevant system. With access to banked and fresh human PBMCs, T cells, B cell, NK cells, and myeloid cell populations, we provide flexible and scalable solutions for immuno-oncology, autoimmune, and inflammation studies.
